Ames test
GenEvolutioN performs, Ames test for Cosmetics, Pharmaceuticals, Chemicals industries, for pure compound, mixtures, plant extracts or impurities (ICH)
Ames test is the gold standard of the bacterial mutation test, well established in OECD 471 guideline.
However, Ames test could be performed using different methods, pre incubation, plate incorporation, micro suspension, in agar, miniaturized, ultra-miniaturized or in fluctuation (liquid form), treat & wash etc.
Micronucleus test
In vitro using whole blood (Human Lymphocyte) in compliance with OECD 487 or superseed by ICH guideline depending on the characteristics of test item. Also available for screening.
QUANTITY
Only 0,1 mg of test article for 5 OECD strains in 2 conditions with or without metabolic activation
INNOVATION
Available only with GenEvolutioN
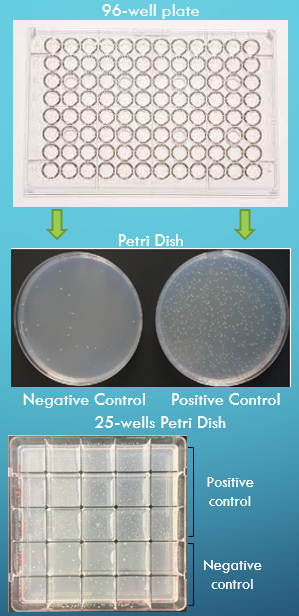
For screening
Non GLP
ACCURACY
Sensitivity 85%
Specificity 91%
Accuracy 87%
Prediction for positive response 94%
Other services
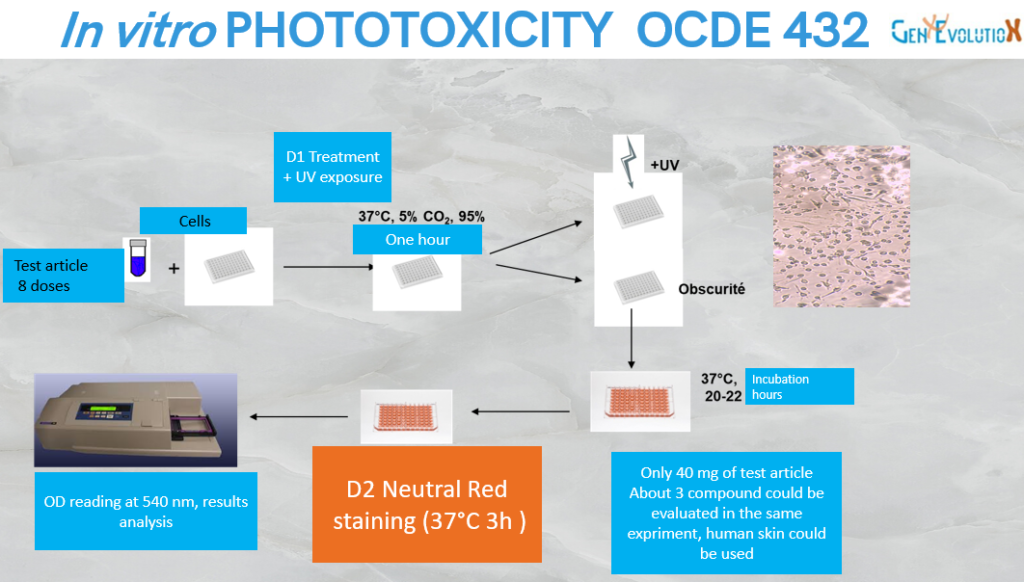
Phototoxicity test
In compliance with OECD 432, on 3T3 cell line
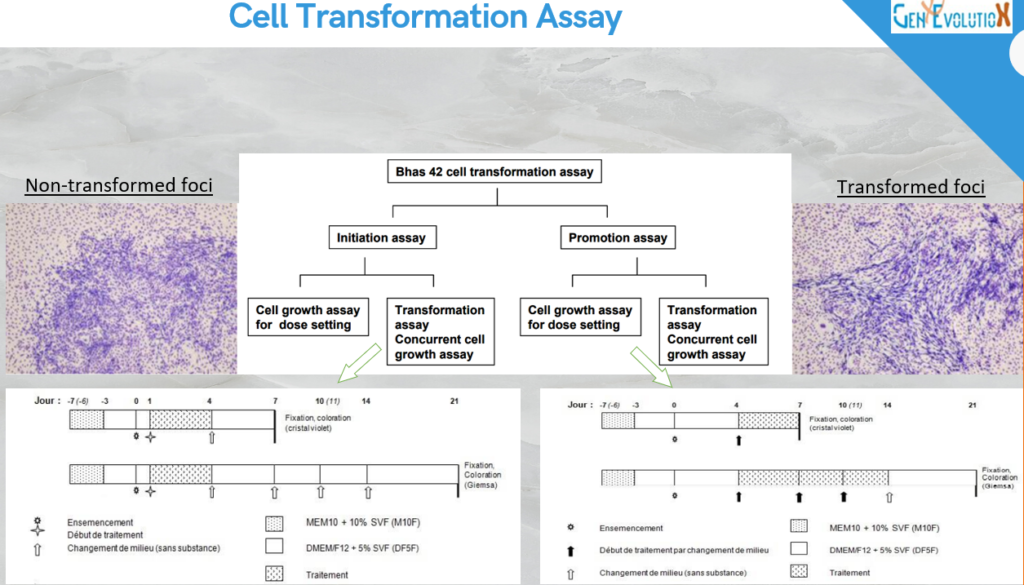
CTA test
Under developing for detection of non genotoxic carcinogenic compound